The Client
The Croatian Institute of Transfusion Medicine collects blood, prepares blood components, tests, stores and distributes approximately 60% of the whole blood supply in Croatia and by this represents the majority of the nation’s blood programs. Croatian Institute of Transfusion Medicine is also the Reference Centre for Transfusion Medicine to the Ministry of Health.
Blood Depot Administration
- 150,000 blood units/year
- Erythorzythen, Octaplas, Quarantan, Thrombozyten, Granulozyten, Stem Cells, Do-norlymphozyten
Laboratory
- Rhesus Lab: > 150, 000 examinations – OR –
- Thrombozythen Lab: > 2.000 examinations – OR –
- TFM Lab: > 50.000 examinations
Blood Production
- Blood product & tissue depot > 150.000 – AND –
- Donations, withdrawals, productions > 5.000
The Trasfusion Medicine network includes:
- Croatian Institute of Transfusion Medicine (e-Delphyn APP/DB host)
- 4 Regional Institutes for Transfusion Medicine
- 5 Local Organization Units for Transfusion Medicine
- 29 Hospital Units for Transfusion Medicine
Scenario
Croatia is a country in eastern Europe with a population of about 4,500,000 inhabitants, and since July 1, 2013 is a member state of the European Union.
One of the requirements demanded of Croatia by the European Union was to adapt its blood donation system to European standards.
These standards require all European blood banks to conduct a comprehensive analysis of donated blood in order to verify that donors do not have any infectious diseases, such as Hepatitis B, Hepatitis C, HIV or Syphilis. Only blood free of any infectious disease can be transfused to patients who need it.
In addition, it is essential that blood banks implement tools that guarantee the correct transfusion of the most compatible blood for each patient.
To meet these requirements, one of the necessary elements has been to install an information system capable of efficiently and safely managing all blood donations in the country. To carry out this ambitious project at the national level, Hemasoft Software was chosen, because it was developed entirely with Web technology, which allows its use on any platform, operating system and its management from any device,
from a PC to a tablet or smart phone.
Hemasoft’s implementation proposal was based on five key elements:
1. organizational management
2. implementation of quality standards
3. documentation
4. training of staff and traceability
After two years of work in collaboration with the National Institute of Blood Transfusion of Croatia, the Blood Banks in the country, together with the 29 Hospitals in it, are already working with eDelphyn software in a centralized way, sharing all the Centers clinical information regarding the blood donors they receive and the patients they transfuse.
In addition, the implementation of the Hemasoft software has meant significant cost savings for the country’s health system, as it has been able to optimize the management of blood warehouses distributed throughout its geography.
The challenges
Although the eDelphyn product proved to be the ideal utility for this project according to its ability to manage centralized and complex environments, the project required an investment with the right mix of resources — including support at the highest levels, leadership, and staff education and empowerment.
While no two institutions are alike, there are many common challenges to overcome through the experience and expertise of Hemasoft´s team, but others challenges are specific to each project that are brought to fruition thanks to eDelphyn base technology and its great capacity and flexibility in terms of configuration.
The solution
eDelphyn Suite Modules installed:
- Blood Donor management
- Transfusion management
- Clinical Lab management (LIS)
- Cell Therapy
Hemasoft’s solution eDelphyn Suite (v.8.0) is installed for being used as a Multi-Centre application enabling 300 concurrence users to enter and to share information among different Centres on real time, providing access to information anytime, anywhere and on a variety of mobile devices.
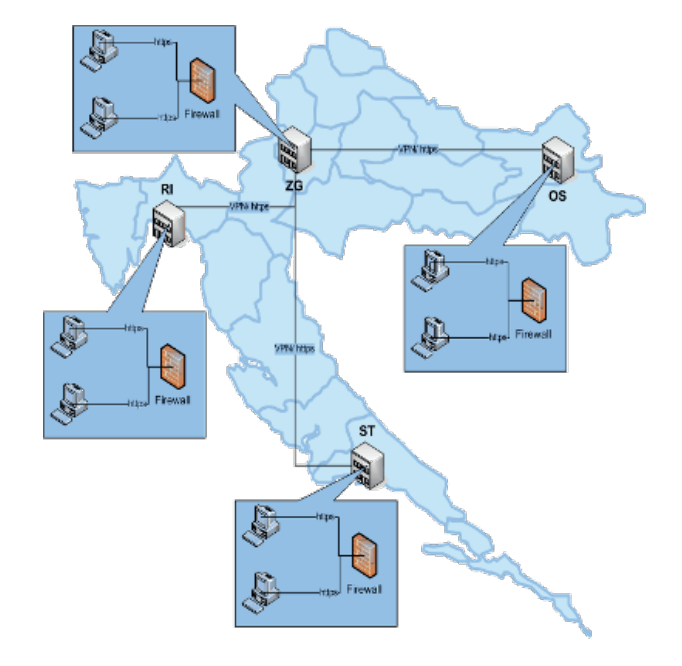
Delivery, installation and putting into operation done in four steps:
Step 1
eDelphyn installation at The National Institute for Transfusion Medicine of Zagreb (HZTM).
Step 2
Four (4) Regional Institutes for Transfusion Medicine (Osijek, Rijeka, Split and Varazdin).
Step 3
Five (5) Local Organization Units for Transfusion Medicine
(Dubrovnik, Pula, Sl. Brod, Zadar and Pozega).
Step 4
Twenty-nine (29) Hospital Units for Transfusion Medicine.
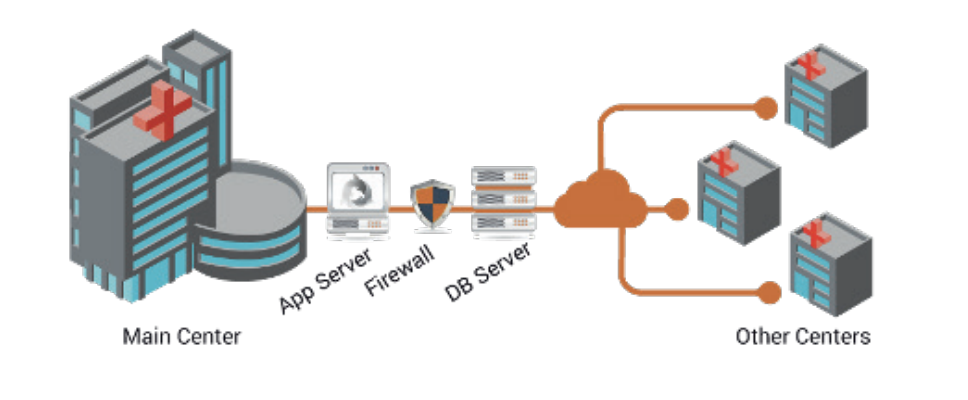
Benefits
The Croatian National Transfusion Service sets out a standard international system, improvement of blood-letting process, obtainment of precise and complete management reports, creation of a centralized information bank and settling the affairs of all computer matters related to blood-letting and distribution of blood.
eDelphyn Software also allows guaranteeing to the Croatian National Transfusion Service a comprehensive automation based on ICCBBA, ISBT128 standard for the purpose of mechanization of its technical operation turnover throughout the country, providing a suitable ground in the direction of having fast, safe and secure access to information from different geographical regions of country and other parts of the world in accordance with international standards.
Additionally, this centralized way of managing all donor and patient information at the national level through the eDelphyn Software, has brought important clinical improvements in the treatment of patients, as hospitals already use tablet-type mobile devices to ensure correct transfusion. of blood to the correct patient, reducing human error to practically zero.
Next Steps
Hemasoft’s projects with its clients are long-term, due to new integrations with new clinical instruments whose data is also susceptible to being managed by the eDelphyn environment, as well as the possibility of adding other departments in the same environment, thus, accessing other brand products, such as the solution for Cell Therapy Labs.


